One of the questions I get asked the most these days is when the world will be able to go back to the way things were in December before the coronavirus pandemic. My answer is always the same: when we have an almost perfect drug to treat COVID-19, or when almost every person on the planet has been vaccinated against coronavirus.
这些天我被问得最多的一个问题是:世界何时才能回到去年12月新冠病毒大流行之前的状态?我的答案始终如一:当我们得到一种近乎完美的特效药的时候,或者当地球上几乎所有人都接种了新冠疫苗的时候。
The former is unlikely to happen anytime soon. We’d need a miracle treatment that was at least 95 percent effective to stop the outbreak. Most of the drug candidates right now are nowhere near that powerful. They could save a lot of lives, but they aren’t enough to get us back to normal.
前者不太可能在短期内实现。我们需要一种有效率达到95%的疗法来遏制疫情。就目前而言,大多数候选药物的效力都远没有那么强大。它们可以挽救很多生命,但它们不足以让我们恢复正常生活。
Which leaves us with a vaccine.
所以,我们只能寄希望于疫苗了。
Humankind has never had a more urgent task than creating broad immunity for coronavirus. Realistically, if we’re going to return to normal, we need to develop a safe, effective vaccine. We need to make billions of doses, we need to get them out to every part of the world, and we need all of this happen as quickly as possible.
形成广泛的新冠病毒免疫力已经成为人类最为紧迫的任务。实际上,如果想要恢复正常生活,我们就需要开发出一种安全有效的疫苗,需要生产数十亿剂并供应到世界各地,并且这一切需要尽快实现。
That sounds daunting, because it is. Our foundation is the biggest funder of vaccines in the world, and this effort dwarfs anything we’ve ever worked on before. It’s going to require a global cooperative effort like the world has never seen. But I know we’ll get it done. There’s simply no alternative.
这听起来令人气馁,因为确实很难。盖茨基金会是世界上最大的疫苗资助者,但我们这次所面临的挑战远远大于先前从事的任何工作。这需要全球开展前所未有的通力合作。但我知道我们终将成功。除此之外别无选择。
Here’s what you need to know about the race to create a COVID-19 vaccine.
新冠疫苗的开发工作正与时间赛跑,以下是你需要知道的事。
The world is creating this vaccine on a historically fast timeline.
世界正以前所未有的速度开发新冠肺炎疫苗
Dr. Anthony Fauci has said he thinks it’ll take around eighteen months to develop a coronavirus vaccine. I agree with him.
安东尼·福奇博士认为大约需要18个月才能开发出新冠肺炎疫苗,我同意他的观点。
Although eighteen months sounds like a long time, this would be the fastest scientists have created a new vaccine. Development usually takes around five years. Once you pick a disease to target, you have to create the vaccine and test it on animals. Then you begin testing for safety and efficacy in humans.
虽然18个月听起来很长,但这将是科学家们迄今为止开发速度最快的新疫苗。开发疫苗通常需要五年左右的时间,一旦你选择了一种疾病作为目标,你就必须研制出疫苗并在动物身上进行试验。接下来,你可以开展人体试验,测试疫苗的安全性和有效性。
Safety and efficacy are the two most important goals for every vaccine. Safety is exactly what it sounds like: is the vaccine safe to give to people? Some minor side effects (like a mild fever or injection site pain) can be acceptable, but you don’t want to inoculate people with
something that makes them sick.
安全性和有效性是每种疫苗最重要的两个目标。安全性就是它的字面意思:给人们接种这个疫苗安全吗?一些轻微的副作用(如轻微发烧或接种部位的疼痛)是可以被接受的,但是你不希望人们因为接种疫苗而生病。
Efficacy measures how well the vaccine protects you from getting sick. Although you’d ideally want a vaccine to have 100 percent efficacy, many don’t. For example, this year’s flu vaccine is around 45 percent effective.
有效性衡量的是疫苗对你的保护效力。尽管理想情况下你希望疫苗有100%的效力,但是很多疫苗并没有这么完美。例如,今年流感疫苗的有效率约为45% 。
To test for safety and efficacy, every vaccine goes through three phases of trials:
为了测试疫苗的安全性和有效性,每种疫苗都要经过三个阶段的临床试验:
Phase oneis the safety trial. A small group of healthy volunteers gets the vaccine candidate. You try out different dosages to create the strongest immune response at the lowest effective dose without serious side effects.
一期临床试验是安全试验,由一小组健康的志愿者接种候选疫苗。你可以尝试使用不同的剂量,目的是用最低的有效剂量产生最强的免疫应答却又不会出现严重副作用。
Once you’ve settled on a formula, you move onto phase two, which tells you how
well the vaccine works in the people who are intended to get it. This time, hundreds of people get the vaccine. This cohort should include people of different ages and health statuses.
在确定一套方案后,你会进入二期临床试验,它将会告诉你疫苗作用于接种人群的效果如何。这一次,数百人将接种疫苗,这个群体应该包括不同年龄和不同健康状况的人。
Then, in phase three, you give it to thousands of people. This is usually the longest phase, because it occurs in what’s called “natural disease conditions.” You introduce it to a sufficient number of people that are likely already at the risk of infection by the target pathogen, and then wait and see if the vaccine reduces how many people get sick.
接着在三期临床试验中,你会让数千人进行接种。这通常是最长的一个阶段,因为它发生在所谓的“自然疾病条件”下。你给足够多的可能已经暴露在目标病原体感染风险下的人接种疫苗,然后看疫苗能多大程度减少患病人数。
After the vaccine passes all three trial phases, you start building the factories to manufacture it, and it gets submitted to the WHO and various government agencies for approval.
在疫苗通过上述三期临床试验后,你还需要有生产疫苗的工厂,并将疫苗提交到世界卫生组织和各个政府机构进行审批。
This process works well for most vaccines, but the normal development timeline isn’t good enough right now. Every day we can cut from this process will make a huge difference to the world in terms of saving lives and reducing trillions in economic damage.
这个过程适用于大多数疫苗,但常规的开发时间表显然不适合新冠疫苗。我们从这一过程中节省下来的每一天,都将对世界产生巨大影响,不仅能挽救生命,而且可以减少数万亿的经济损失。
So, to speed up the process, vaccine developers are compressing the timeline. This graphic shows how:
因此,为了加快这一进程,疫苗开发人员正在压缩时间,从下面这张图可以看到我们是如何做到的。
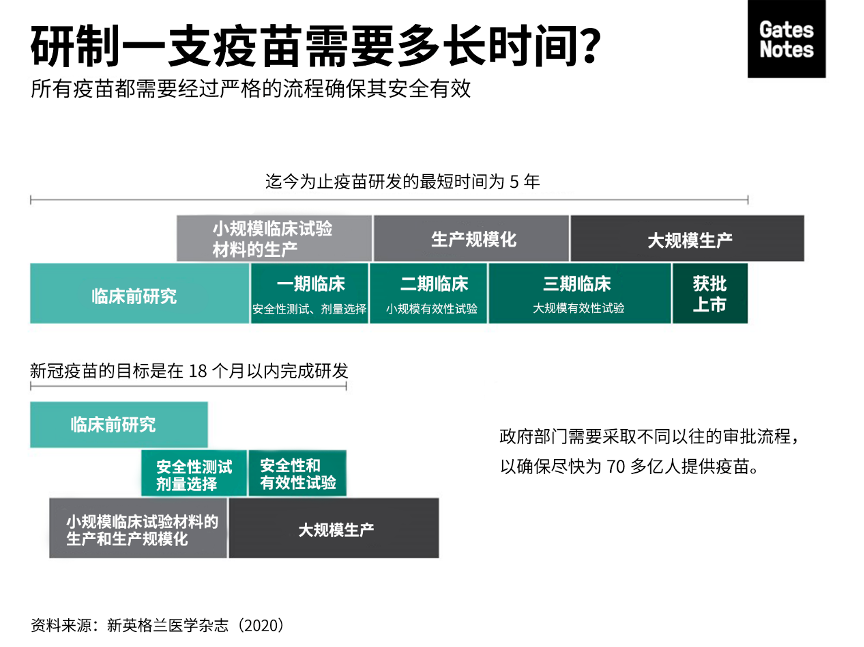
In the traditional process, the steps are sequential to address key questions and unknowns. This can help mitigate financial risk, since creating a new vaccine is expensive. Many candidates fail, which is why companies wait to invest in the next step until they know the previous step was successful.
在传统过程中,这些步骤是按顺序进行的,从而解决关键问题和未知因素。同时这样也有助于降低金融风险,因为开发一种新疫苗耗资不菲。许多候选疫苗最终都失败了,这就是为什么企业需要等到明确上一步成功后才会继续投资下一步工作。
For COVID-19, financing development is not an issue. Governments and other organizations (including our foundation and an amazing organization called the Coalition for Epidemic Preparedness Innovations) have made it clear they will support whatever it takes to find a vaccine. So, scientists are able to save time by doing several of the development steps at once. For example, the private sector, governments, and our foundation are going to start identifying facilities to manufacture different potential vaccines. If some of those facilities end up going unused, that’s okay. It’s a small price to pay for getting ahead on production.
对于新冠疫苗而言,开发资金不是问题。各国政府和其他机构——包括我们的基金会和一个叫做“流行病防范创新联盟”(Coalition for Epidemic Preparedness Innovations,CEPI)的了不起的组织——已经明确表示,将不惜一切代价支持疫苗开发。因此,科学家们可以通过平行进行几个开发步骤来节省时间。例如,私营部门、政府和我们的基金会将开始确定生产不同潜在疫苗的设施。即使部分设施最终不被使用,那也没关系。为了缩短疫苗生产进程,这只是一个小小的代价。
Fortunately, compressing the trial timeline isn’t the only way to take a process that usually takes five years and get it done in 18 months. Another way we’re going to do that is that by testing lots of different approaches at the same time.
所幸,压缩临床试验时间并不是将疫苗开发周期从五年缩短至18个月的唯一方法。我们即将采取的另一个措施是同时试验许多不同的方法。
There are dozens of candidates in the pipeline
数十个候选疫苗正在筹备中
As of April 9, there are 115 different COVID-19 vaccine candidates in the development pipeline. I think that eight to ten of those look particularly promising. (Our foundation is going keep an eye on all the others to see if we missed any that have some positive characteristics, though.)
截至4月9日,已经有115个不同的新冠肺炎候选疫苗正在开发中。我认为其中的8到10个看起来很有前景。(尽管如此,我们的基金会还是会密切关注所有其他疫苗,以免遗漏了那些具有技术潜力的疫苗。)
The most promising candidates take a variety of approaches to protecting the body against COVID-19. To understand what exactly that means, it’s helpful to remember how the human immune system works.
最有前景的候选疫苗采取了各种方法来保护人体免受新冠病毒的侵害。这句话究竟意味着什么?让我们先来了解人类免疫系统是如何工作的。
When a disease pathogen gets into your system, your immune system responds by producing antibodies. These antibodies attach themselves to substances called antigens on the surface of the microbe, which sends a signal to your body to attack. Your immune system keeps a record of every microbe it has ever defeated, so that it can quickly recognize and destroy invaders before they make you ill.
当一种病原体进入人体,你的免疫系统就会产生抗体。这些抗体附着在微生物表面称为抗原的物质上,向你的身体发出攻击信号。你的免疫系统会记录它曾经击败过的每一种微生物,这样就能在入侵者使你生病之前迅速识别并消灭它们。
Vaccines circumvent this whole process by teaching your body how to defeat a pathogen without ever getting sick. The two most common types—and the ones you’re probably most familiar with—are inactivated and live vaccines. Inactivated vaccines contain pathogens that have been killed. Live vaccines, on the other hand, are made of living pathogens that have been weakened (or “attenuated”). They’re highly effective but more prone to side effects than their inactivated counterparts.
疫苗能够避免这整个过程,它能教会你的身体如何在不生病的情况下战胜病原体。两种最常见的类型(也是你可能最熟悉的类型)是灭活疫苗和活疫苗。灭活疫苗含有被杀死的病原体。而活疫苗是由毒性被减弱(或“减毒”)的活病原体制成的,它们非常有效,但相比同类的灭活疫苗更容易产生副作用。
Inactivated and live vaccines are what we consider “traditional” approaches. There are a number of COVID-19 vaccine candidates of both types, and for good reason: they’re well-established. We know how to test and manufacture them.
灭活疫苗和减毒活疫苗是我们所认为的“传统”方法。这两种类型的新冠候选疫苗有很多。原因很简单:它们的技术都很成熟。我们知道如何测试和制造它们。
The downside is that they’re time-consuming to make. There’s a ton of material in each dose of a vaccine. Most of that material is biological, which means you have to grow it. That takes time, unfortunately.
但它们的缺点是制造起来很费时间。每剂疫苗都需要大量原材料,其中大部分是生物材料,这意味着你需要时间来培养它们。
That’s why I’m particularly excited by two new approaches that some of the candidates are taking: RNA and DNA vaccines. If one of these new approaches pans out, we’ll likely be able to get vaccines out to the whole world much faster. (For the sake of simplicity, I’m only going to explain RNA vaccines. DNA vaccines are similar, just with a different type of genetic material.)
这就是我为什么对目前一些候选疫苗采取的两种新方法感到特别兴奋:RNA疫苗和DNA疫苗。如果任何一个新方法获得成功,我们都能更快地将疫苗提供给全世界。(简单起见,我在此只解释一下RNA疫苗, DNA疫苗的原理是近似的,只是使用不同的遗传物质。)
Our foundation—both through our own funding and through CEPI—has been supporting the development of an RNA vaccine platform for nearly a decade. We were planning to use it to make vaccines for diseases that affect the poor like malaria, but now it’s looking like one of the most promising options for COVID. The first candidate to start human trials was an RNA vaccine created by a company called Moderna.
我们的基金会近十年来一直通过我们自己的资金和CEPI支持RNA疫苗平台的开发。我们原先计划用它来开发疫苗抗击那些影响贫困人群的疾病(例如疟疾),但现在看起来它可能是抗击新冠肺炎最有前景的方案之一。第一个开始人体试验的候选疫苗是由一家名为Moderna的公司制造的RNA疫苗。
Here’s how an RNA vaccine works: rather than injecting a pathogen’s antigen into your body, you instead give the body the genetic code needed to produce that antigen itself. When the antigens appear on the outside of your cells, your immune system attacks them – and learns how to defeat future intruders in the process. You essentially turn your body into its own vaccine manufacturing unit.
RNA疫苗的工作原理不是将病原体的抗原注射到人体内,而是给人体提供产生抗原所需的遗传密码。当抗原出现在细胞外部时,你的免疫系统就会攻击它们,并在此过程中学习如何打败未来的入侵者。本质上就是把人体变成自己的疫苗生产部门。
There’s a catch, though: we don’t know for sure yet if RNA is a viable platform for vaccines. Since COVID would be the first RNA vaccine out of the gate, we have to prove both that the platform itself works and that it creates immunity. It’s a bit like building your computer system and your first piece of software at the same time.
不过,还有一个问题:我们目前还不能确定RNA是否是开发疫苗的可行平台。由于新冠RNA疫苗将是第一个上市的RNA疫苗,我们必须证明RNA平台本身可行并可以产生免疫力。这有点像同时构建计算机系统和系统里的第一个软件。
Even if an RNA vaccine continues to show promise, we still must continue pursuing the other options. We don’t know yet what the COVID-19 vaccine will look like. Until we do, we have to go full steam ahead on as many approaches as possible.
因此,即便RNA疫苗有望成功,我们仍必须继续寻求其他可能的方法。我们还不知道新冠疫苗会是什么样,在此之前,我们必须全力以赴,尝试尽可能多的方法。
It might not be a perfect vaccine yet—and that’s okay.
它可能还不是完美的疫苗,但是没关系
The smallpox vaccine is the only vaccine that’s wiped an entire disease off the face of the earth, but it’s also pretty brutal to receive. It left a scar on the arm of anyone who gets it. One of out every three people who got it had side effects bad enough to keep them home from school or work. A small—but not insignificant—number developed more serious reactions.
天花疫苗是世界上唯一一个将一种疾病从地球上根除的疫苗,但接种天花疫苗的过程有些痛苦。它会在接种者的手臂上留疤,每三个人中就会有一人因接种天花疫苗产生的副作用而只能暂时呆在家中,无法上学或上班。其中还会有不可忽视的小部分人会产生出更严重的反应。
The smallpox vaccine was far from perfect, but it got the job done. The COVID-19 vaccine might be similar.
天花疫苗远非完美,但它完成了使命。新冠疫苗可能也会如此。
If we were designing the perfect vaccine, we’d want it to be completely safe and 100 percent effective. It should be a single dose that gives you lifelong protection, and it should be easy to store and transport. I hope the COVID-19 vaccine has all of those qualities, but given the timeline we’re on, it may not.
如果要设计一种完美的疫苗,我们希望它是百分百安全且有效。你只需要接种一剂,就可以得到终生免疫保护。而且疫苗的储存和运输也应该很方便。我希望新冠疫苗将会具备所有这些特性,然而鉴于我们的时间表,可能无法面面俱到。
The two priorities, as I mentioned earlier, are safety and efficacy. Since we might not have time to do multi-year studies, we will have to conduct robust phase 1 safety trials and make sure we have good real-world evidence that the vaccine is completely safe to use
正如我之前提到的,疫苗的两个重点是安全性和有效性。由于我们可能没有时间进行多年的研究,我们将不得不进行强有力的一期安全试验以及良好的真实世界数据,并确保我们有充分证据表明疫苗是可以安全使用。
We have a bit more wiggle room with efficacy. I suspect that vaccine that is at least 70 percent effective will be enough to stop the outbreak. A 60 percent effective vaccine is useable, but we might still see some localized outbreaks. Anything under 60 percent won’t likely create enough herd immunity to stop the virus.
在有效性方面,我们还有一些余地。我估计有效性至少达到70%的疫苗就足以遏制疫情。有效性达到60%的疫苗是可用的,但可能仍然会看到局部暴发的疫情。任何低于60%有效性的疫苗都不可能产生足够的群体免疫来阻止病毒的传播。
The big challenge will be making sure the vaccine works well in older people. The older you are, the less effective vaccines are. Your immune system—like the rest of your body—ages and is slower to recognize and attack invaders. That’s a big issue for a COVID-19 vaccine, since older people are the most vulnerable. We need to make sure they’re protected.
最大的挑战是确保疫苗对老年人也有很好的效果。年龄越大,疫苗的效果越弱。你的免疫系统,如同你身体其他部分一样,也会随着年龄的增长而衰老,识别和攻击入侵者的速度也会变慢。这对新冠疫苗来说是个大问题,因为老年人也是最容易感染新冠病毒的人群,我们需要确保他们得到保护。
The shingles vaccine—which is also targeted to older people—combats this by amping up the strength of the vaccine. It’s possible we do something similar for COVID, although it might come with more side effects. Health authorities could also ask people over a certain age to get an additional dose.
作为同样针对老年人的疫苗,带状疱疹疫苗通过增加疫苗的效力来解决上述问题。针对新冠病毒,我们可以做类似的事情,尽管这可能带来更多副作用。卫生部门也可以让超过一定年龄的人群多接种一剂。
Beyond safety and efficacy, there are a couple other factors to consider:
除了安全性与有效性外,还有以下几个因素需要考虑:
How many doses will it be?A vaccine you only get once is easier and quicker to deliver. But we may need a multi-dose vaccine to get enough efficacy.
要接种多少剂?只需要接种一剂的疫苗,相对更便捷、更快速。但我们可能需要接种多剂的疫苗才能获得足够的效力。
How long does it last?Ideally, the vaccine will give you long-lasting protection. But we might end up with one that only stops you from getting sick for a couple months (like the seasonal flu vaccine, which protects you for about six months). If that happens, the short-term vaccine might be used while we work on a more durable one.
疫苗效力可以维持多久?理想情况下,疫苗可以提供持久的保护。但最终我们可能会得到一种只能为你提供几个月保护的疫苗 (与季节性流感疫苗类似,免疫时效大约为6个月左右)。如果真是这样,我们很可能在研究更持久疫苗的同时,先使用短期有效的疫苗。
How do you store it? Many common vaccines are kept at 4 degrees C. That’s around the temperature of your average refrigerator, so storage and transportation is easy. But RNA vaccines need to be stored at much colder temperature—as low as -80 degrees C—which will make reaching certain parts of the world more difficult.
疫苗如何储存?许多常见的疫苗需要储藏在4摄氏度的条件下,这大约是一般冰箱的温度,所以储藏与运输也相对简单。但RNA疫苗需要储藏在更低的温度下,可能低至零下80摄氏度,将其运输到世界某些地方会变得更加困难。
My hope is that the vaccine we have 18 months from now is as close to “perfect” as possible. Even if it isn’t, we will continue working to improve it. After that happens, I suspect the COVID-19 vaccine will become part of the routine newborn immunization schedule.
我希望18个月后诞生的新冠疫苗尽可能地接近“完美”。就算不是,我们仍将继续努力改进它。在此之后,我认为新冠疫苗将成为新生儿常规免疫计划中的一部分。
Once we have a vaccine, though, we still have huge problems to solve. That’s because…
就算有了疫苗,我们仍需解决一些重要问题,那是因为……
We need to manufacture and distribute at least 7 billion doses of the vaccine.
我们需要生产和分发至少70亿剂疫苗
In order to stop the pandemic, we need to make the vaccine available to almost every person on the planet. We’ve never delivered something to every corner of the world before. And, as I mentioned earlier, vaccines are particularly difficult to make and store.
为了遏制大流行病,我们需要确保地球上几乎每一个人都能接种疫苗。将某个东西送达到世界上的每一个角落,这在过去从未发生过。同时,正如我之前提到的,疫苗的生产和储藏也非常困难。
There’s a lot we can’t figure out about manufacturing and distributing the vaccine until we know what exactly we’re working with. For example, will we be able to use existing vaccine factories to make the COVID-19 vaccine?
在明确最终会使用哪种疫苗之前,我们无法确定如何生产和分发疫苗。例如,我们能否利用现有的疫苗工厂生产新冠疫苗?
What we can do now is build different kinds of vaccine factories to prepare. Each vaccine type requires a different kind of factory. We need to be ready with facilities that can make each type, so that we can start manufacturing the final vaccine (or vaccines) as soon as we can. This will cost billions of dollars. Governments need to quickly find a mechanism for making the funding for this available. Our foundation is currently working with CEPI, the WHO, and governments to figure out the financing.
我们现在能做的是为各种不同类型的疫苗建造工厂。每一种类型的疫苗需要不同的生产工厂。我们需要准备好生产各种疫苗的设备,从而确保当一种或多种疫苗研发完成后尽快开始生产。这将耗资数十亿美元,各国政府需要迅速找到一种机制来为此提供资金。盖茨基金会正在与CEPI、世界卫生组织及各国政府一同筹措资金。
Part of those discussions center on who will get the vaccine when. The reality is that not everyone will be able to get the vaccine at the same time. It’ll take months—or even years—to create 7 billion doses (or possibly 14 billion, if it’s a multi-dose vaccine), and we should start distributing them as soon as the first batch is ready to go.
还有一些讨论集中在谁将于何时接种疫苗。现实情况是,我们无法让所有人同时接种疫苗,而是需要数月甚至数年的时间才能制造出70亿剂疫苗(如果是接种多剂疫苗,甚至可能是140亿剂),我们应该在有了第一批疫苗之后立即开始进行分发。
Most people agree that health workers should get the vaccine first. But who gets it next? Older people? Teachers? Workers in essential jobs?
多数人同意卫生工作者应该优先接种疫苗。但接下来该为谁接种呢?老年人?教师?在关键岗位工作的人?
I think that low-income countries should be some of the first to receive it, because people will be at a much higher risk of dying in those places. COVID-19 will spread much quicker in poor countries because measures like physical distancing are harder to enact. More people have poor underlying health that makes them more vulnerable to complications, and weak health systems will make it harder for them to receive the care they need. Getting the vaccine out in low-income countries could save millions of lives. The good news is we already have an organization with expertise about how to do this in Gavi, the Vaccine Alliance.
我认为低收入国家的人民应该在第一批接种疫苗,因为他们面临着更高的死亡风险。由于物理隔离等措施更难以实施,新冠疫情会在贫穷国家传播得更快。更多人的基础健康情况不佳,使他们更易出现并发症,而在薄弱的卫生系统中,他们很难获得所需的治疗。为低收入国家提供疫苗能够挽救数百万人的生命。好消息是,我们已经有全球疫苗免疫联盟(Gavi)这样的专业机构从事这方面的工作。
With most vaccines, manufacturers sign a deal with the country where their factories are located, so that country gets first crack at the vaccines. It’s unclear if that’s what will happen here. I hope we find a way to get it out on an equitable basis to the whole world. The WHO and local health authorities will need to develop a distribution plan once we have a better understanding of what we’re working with.
对于大多数疫苗而言,疫苗生产者会与工厂所在的国家签署协议,确保该国率先获得疫苗。目前还不清楚这种情况是否会发生。我希望我们可以找到一种方式,在公平的基础上向全世界提供疫苗。一旦我们对于现在的工作进展更加明朗,世界卫生组织和地方卫生部门将需要制定一个疫苗分配计划。
Eventually, though, we’re going to scale this thing up so that everybody on the planet can get the vaccine. And then, we’ll be able to get back to normal—and to hopefully make decisions that prevent us from being in this situation ever again.
最终,我们会扩大生产规模,让地球上每一个人都能接种疫苗。之后,我们就能恢复正常生活,并有望通过明智的决策避免再次陷入相同的困境。
It might be a bit hard to see right now, but there is a light at the end of the tunnel. We’re doing the right things to get a vaccine as quickly as possible. In the meantime, I urge you to continue following the guidelines set by your local authorities. Our ability to get through this outbreak will depend on everyone doing their part to keep each other safe.
虽然前路迷茫,但光明就在前方。我们正在采取正确的做法,从而尽快获得有效疫苗。与此同时,我迫切期望大家能继续遵循当地卫生部门的指导。我们能否平安度过这次疫情,取决于每个人能否各尽其责以确保自己和他人的安全。